The US Court of Appeals for the Federal Circuit affirmed the Patent Trial & Appeal Board, holding that the Board did not err in finding certain challenged claims nonobvious and not unpatentable based on a showing of several objective criteria of nonobviousness and a nexus of the evidence to a commercial product embodying the claimed invention. Medtronic, Inc. v. Teleflex Innovations S.A.R.L., Case No. 21-2357 (Fed. Cir. June 05, 2023) (Moore, C.J.; Lourie, Dyk, JJ.) and Medtronic, Inc. v. Teleflex Innovations S.A.R.L., Case No. 21-2359 (Fed. Cir. June 05, 2023) (Moore, C.J.; Lourie, Dyk, JJ.)
Teleflex developed and patented a novel catheter-based stenosis intervention system that successfully mitigated long-standing risks intrinsic to existing catheter-based intervention systems, in particular damage to the coronary artery from guide catheter dislodgement or a catheter’s distal tip (i.e., the end of the catheter farthest from the insertion site). The preferred embodiments incorporated into Teleflex’s extremely successful GuideLiner products comprised a proximal substantially rigid portion (yellow), a reinforced portion (blue) and a distal flexible tip (pink), as illustrated below.
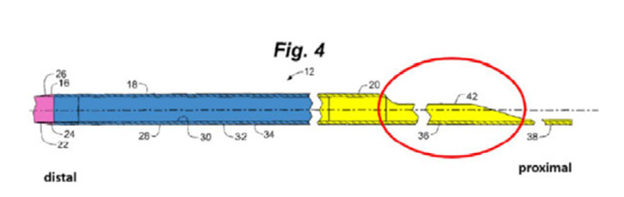
The catheters were sized so they could be inserted through standard guide catheters and thus were coined guide extension catheters. This innovative nesting feature increased guide catheter backup support while the guide extension catheter’s soft distal end was less likely to cause tissue damage once deeply inserted into patients. Teleflex’s guide extension catheters also were optimized for receiving interventional cardiological devices. This optimized function was a combination of the catheter’s coaxial lumen, that lumen’s diameter being no more than one French (i.e., 1/3 mm) less than the diameter of the guide catheter, and a proximal side opening that featured a double incline design like that illustrated above.
Teleflex’s GuideLiner was introduced in 2009 and enjoyed “undisputed commercial success and industry praise.” In 2019, Medtronic introduced its competing guide extension catheter (Telescope) and filed six inter partes review (IPR) petitions against Teleflex’s extension guide catheter family. Three of Medtronic’s petitions asserted that the challenged claims in three of Teleflex’s patents were obvious over the evacuation sheath assembly with a distal side opening used to aspirate embolic material while occluding blood flow using sealing balloons disclosed in a prior art reference (Ressemann). The other three petitions challenged claims of the other Teleflex patents as being obvious over a support catheter for delivering angioplasty balloons disclosed in a prior art reference (Kontos).
Medtronic specifically asserted that the following three elements of Teleflex’s claimed catheters were obvious:
- A proximal side opening. Medtronic argued that it would have been obvious to replace the proximal funnel structure of Kontos’s support catheter with the distal side opening of Ressemann’s evacuation sheath assembly.
- A catheter diameter that is no more than one French less than a corresponding guide catheter. Medtronic argued that in view of prior art mother-and-child dual catheter systems in which the child catheter’s diameter is no more than one French less than the mother’s diameter, it would have been obvious to further modify Kontos’s support catheter or Ressemann’s evacuation sheath assembly to be no more than one French less in diameter than a corresponding guide catheter.
- A proximal double incline. Medtronic argued that it would have been obvious to further modify the proximal end of Kontos’s support catheter or Ressemann’s evacuation sheath assembly to comprise the double-inclined distal tip of the suction catheter disclosed in yet another prior art reference (Kataishi).
The Board initiated all six petitions and issued final written decisions in each, holding some claims unpatentable and others not. The Board also granted Teleflex’s motions to amend certain challenged claims and held that these amended claims were not unpatentable. In holding that certain challenged claims were not unpatentable, the Board rejected Medtronic’s allegations of obviousness by crediting Teleflex’s experts’ testimonies that those of ordinary skill in the art would not be motivated to modify Kontos’s support catheter or Ressemann’s evacuation sheath assembly to achieve the claimed inventions. The Board credited the objective indicia of nonobviousness showing that Teleflex offered concerning its GuideLiner products (e.g., commercial success, industry praise, competitor copying, satisfaction of a long-felt need).
Medtronic appealed all six final written decisions, specifically challenging the Board’s holding that some of the challenged claims were nonobvious. The Federal Circuit addressed Medtronic’s challenges in two separate decisions: one directed to the IPRs relying on obviousness based on Kontos (Case 21-2357) and the other on the IPRs arising from Ressemann (Case 21-2359).
Substantial Evidence
While the obviousness issues addressed in the two appeals were not completely overlapping (e.g., Medtronic only appealed the Board’s treatment of Teleflex’s objective indicia of nonobviousness in the Kontos appeal), the Federal Circuit concluded that most of the issues Medtronic raised amounted to disagreements with the Board’s fact-finding, not legal errors. For those issues, the Court held that substantial evidence supported the Board’s decision to credit Teleflex’s experts over Medtronic’s, and thus affirmed the Board’s fact-finding. The Court cited the 1966 Supreme Court decision in Consolo v. Fed. Mar. Comm’n and the 2003 Federal Circuit decision in Velander v. Garner, which respectively held the following:
- “[T]he possibility of drawing two inconsistent conclusions from the evidence does not prevent an administrative agency’s finding from being supported by substantial evidence.”
- “If the evidence will support several reasonable but contradictory conclusions, we will not find the Board’s decision unsupported by substantial evidence simply because the Board chose one conclusion over another plausible alternative.”
The Federal Circuit also dismissed multiple Medtronic assertions that the Board erred because its final written decisions did not include the Board’s rationale for dismissing Medtronic’s “motivation to combine” arguments as “unpersuasive.” While acknowledging that the Board’s written decisions could have been more detailed, the Court found that the Board at least met and sometimes exceeded the standard set forth in the Federal Circuit’s 2015 decision in Ariosa Diagnostics—that the Board’s decision-making path was “reasonably discernable.”
Objective Indicia and Nexus
The Federal Circuit also concluded that the Board correctly found Teleflex’s objective indicia of nonobviousness because there was a nexus between the evidence presented and the claimed subject matter. The Board correctly found a presumption of a nexus between Teleflex’s objective evidence and the claims at issue because the objective evidence was tied to specific products—Teleflex’s GuideLiner—and the Board correctly explained that Medtronic could have rebutted this presumption had it demonstrated that Teleflex’s objective evidence had resulted from features that were known as a combination in the prior art.
The Federal Circuit characterized Medtronic’s challenge to the Board’s nexus finding as merely amounting to another “disagreement with the Board’s fact finding,” not identifying potential legal error. The Court noted that Medtronic characterized the relevant combination of features that attributed to GuideLiner’s commercial success (i.e., its rapid exchange functionality, increased backup support and side opening) as also cumulatively found in Ressemann. The Court explained, however, that there was substantial evidence to credit Teleflex’s characterization that GuideLiner’s success was also attributable to its tubular coaxial lumen that in combination with the side opening facilitated reception of the full array of interventional cardiology devices, and substantial evidence to conclude that the prior art did not teach a coaxial lumen. As the Court explained, “Medtronic could rebut this presumption by showing Teleflex’s objective evidence resulted from features that were known, as a combination, in the prior art rather than the claimed invention as a whole. . . . The Board did not err in finding Medtronic failed to make such a showing here.”
The Federal Circuit next considered Teleflex’s objective evidence, which included copying by competitors, a high level of commercial success, significant industry praise and solving a long-felt need within the medical community. The Court characterized the evidence on these indicia as “strong” and “unusually strong;” sufficient to demonstrate the nonobviousness of all claims at issue.
The Federal Circuit highlighted the critical role Teleflex’s objective evidence played with respect to the claims the Board identified as not unpatentable, claims that included the one-French or double-incline limitations. Regarding those claims, the Board found that Medtronic’s prima facie case for obviousness was “close,” but was overcome by Teleflex’s objective evidence. The Court noted that the Board’s finding of objective evidence was entitled to deference, but in any event, found no reason to question the Board’s position that such strong objective evidence was sufficient to overcome even a “close” case of prima facie obviousness.
Practice Note: Be sure to get all your arguments to the Board as early in an IPR proceeding as possible. The Federal Circuit noted that the Board did not err in refusing to consider Medtronic’s arguments regarding modifications to Kontos’s support catheter or Ressemann’s evacuation sheath assembly (arguments necessary to meet the claimed catheters), since Medtronic first presented its “impermissible hindsight” arguments in its reply brief and the Board was entitled to exercise its discretion to not consider arguments not timely raised.





